We have contributed disease modeling and drug development using disease-specific iPSC lines on ring chromosomes (Bershteyn*, Hayashi* et al., Nature 2014: *equally contributed), Fibrodysplasia Ossificans Progressiva (Hayashi et al PNAS 2016; Matsumoto*, Hayashi* et al., OJRD 2013), Long QT syndromes (Spencer et al., Stem Cell Reports 2014). Our team continues to conduct research projects on basic medicine and drug development using disease-specific iPSC lines provided by RIKEN cell bank.
- We develop the differentiation-induction system toward specific disease-targeted cell types if the induction protocols are not established.
- We identify the abnormal cellular phenotypes recapitulating the disease using the differentiated cells from iPSCs in vitro, by comparing the results between disease-specific iPSCs and healthy-donor iPSCs (or isogenic control iPSCs)
- After we establish the assay system described above, we perform the experiments to search for the responsible genes and screening drug candidates.
Example
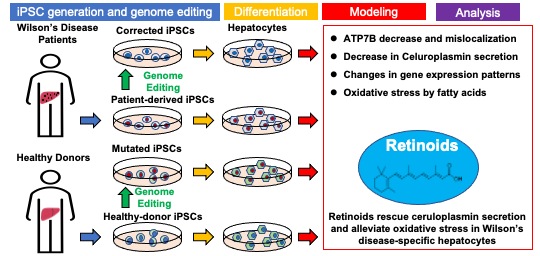
Retinoids rescue ceruloplasmin secretion and alleviate oxidative stress in Wilson’s disease-specific hepatocytesreprogramming to pluripotency
Wilson’s disease (WD) is a copper metabolic disorder caused by a defective ATP7B function. Conventional therapies cause severe side effects and significant variation in efficacy, according to cohort studies. Thus, exploring new therapeutic approaches to prevent progression to liver failure is urgent. To study the physiology and pathology of WD, immortalized cell lines and rodent WD models have been used conventionally; however, a large gap remains among different species as well as in genetic backgrounds among individuals. We generated induced pluripotent stem cells (iPSCs) from 4 WD patients carrying compound heterozygous mutations in the ATP7B gene. ATP7B loss- and gain-of-functions were further manifested with ATP7B-deficient iPSCs and heterozygously corrected R778L WD patient-derived iPSCs using CRISPR-Cas9-based gene editing. Although the expression of ATP7B protein varied among WD-specific hepatocytes differentiated from these iPSCs, the expression and secretion of ceruloplasmin (Cp), a downstream copper carrier in plasma, was consistently decreased in WD-patient-derived and ATP7B-deficient hepatocytes. A transcriptome analysis detected abnormalities in the retinoid signaling pathway and lipid metabolism in WD-specific hepatocytes. Drug screening using WD-patient-derived hepatocytes identified retinoids as promising candidates for rescuing Cp secretion. All-trans retinoic acid (ATRA) also alleviates reactive oxygen species (ROS) production induced by lipid accumulation in WD-specific hepatocytes treated with oleic acid. These patient-derived iPSC-based hepatic models function as effective platforms for the development of potential therapeutics for hepatic steatosis in WD and other fatty liver diseases.